This series of short articles produced by Bro James McCann of the Riverwood ecclesia(au) is a wonderful resource and provide well-reasoned arguments in support of a creation over theistic evolution (Evolutionary creationism).
Amazing Creative Designs
What is one of the simplest substances yet arguably the most important molecule needed for life? What do we use for the most basic functions like drinking or washing ourselves with, to uses like maintaining biological systems, providing a habitat for a multitude of organisms or even regulating global weather and temperature? Dihydrogen monoxide or more commonly, water.
Water is unique in so many ways and without these properties, life would cease to exist. Water is:
- A raw material for many reactions, like photosynthesis
- A solvent that drives our metabolism
- A transport medium, like our blood which is approximately an 80% aqueous suspension
- A lubricant for joints in our body
- A support so that cells maintain their shape
- A coolant as organisms that sweat can regulate their temperature
- A habitat for marine and aquatic organisms
- Global temperature regulator due to its very high heat capacity
But what makes water so amazingly versatile? How is water one of the only substances on earth that are found in all three physical states (solid, liquid and gas)?
It is the properties of water that make it such a unique substance and needful to support life. Compared to many molecules of similar size, shape and bonding water still stands out.
Melting Point and Boiling Point
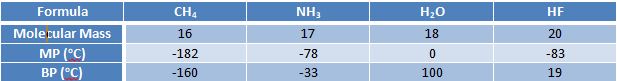
Without such a high MP and BP water would not be able to fulfil half of the uses it is necessary for. Is this coincidence or an accident? Obviously not. In fact, water has much higher than expected values for many properties, including surface tension and viscosity.
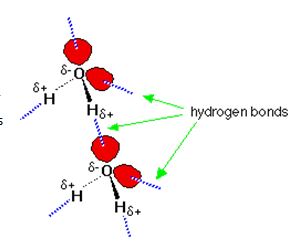
Many of water’s unique properties come from the type of bonds that hold water molecules together. This intermolecular force is called “hydrogen bonding”. It is a special type of bonding that is created because of water’s bent shaped molecule and the way the Oxygen and Hydrogen atoms unequally share electrons. Oxygen loves electrons more than hydrogen, so they spend more time around the oxygen than they do around the hydrogen side of the molecule (creating two poles; one slightly negative and the other slightly positive). This electrostatic attraction then holds the water molecules together.
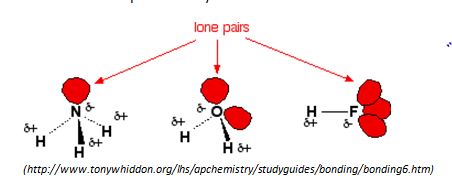
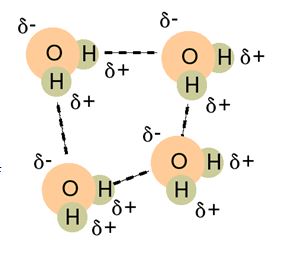 Many substances have hydrogen bonding, see NH3 and HF above. Yet water is still unique. Why?
Many substances have hydrogen bonding, see NH3 and HF above. Yet water is still unique. Why?
Water is considered the perfect hydrogen bonding molecule. This is because there are two lone electron pairs on the Oxygen side of the molecule that can form electrostatic attractions with two hydrogen ends of other molecules, so a 1:1 ratio. This makes the water molecules have 4 strong hydrogen bonds with all sides of the molecule used up effectively.
 (http://www.tonywhiddon.org/lhs/apchemistry/studyguides/bonding/bonding6.htm)
(http://www.tonywhiddon.org/lhs/apchemistry/studyguides/bonding/bonding6.htm)
When you compare this with Ammonia (NH3), the Nitrogen end of the molecule only has one lone pair so it can only make one hydrogen bond with any 3 of the hydrogen from another molecule, so the ratio is 3:1.
This is clearly a wonderful design feature. Chemists see this as the “perfect” model of hydrogen bonding and it is the reason behind many of waters unique but vital properties like:
- Capillary Action – this is the process where water is taken up by the roots of plants and travels up the stem against the force of gravity. Each molecule “pulls” the other up.
- Global temperature regulator – the extensive hydrogen bonding causes water to have significantly high specific heat capacity. So vast quantities of energy can be stored in water. The entire climate is regulated by this process and is the basis of the water cycle.
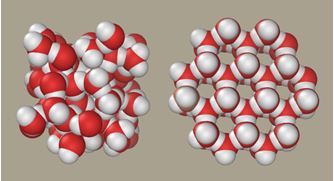 Density – with most substances, the solid is denser than the liquid, making the solid sink in the liquid. However, with water this is the opposite, and vitally so. Due to the hydrogen bonding causing the molecules to arrange themselves in a more structured hexagonal crystal, the solid expands making the density of ice less then water. The ice then floats on top of the water, creating a protective barrier for marine and aquatic animals to live under and a great insulator. If lakes and rivers froze from the bottom up then all life in them would be killed.
Density – with most substances, the solid is denser than the liquid, making the solid sink in the liquid. However, with water this is the opposite, and vitally so. Due to the hydrogen bonding causing the molecules to arrange themselves in a more structured hexagonal crystal, the solid expands making the density of ice less then water. The ice then floats on top of the water, creating a protective barrier for marine and aquatic animals to live under and a great insulator. If lakes and rivers froze from the bottom up then all life in them would be killed.
When researching the properties of water you will often find statements like, “It is lucky for us that ice floats”. However it is clear that such an important feature on which life depends is not an accident but a design feature of an intelligent Creator.
“Does the rain have a father? Who fathered the dew? Whose womb brings forth the ice? Who gives birth to frost out of an empty sky when water solidifies like stone and the surface of the deepest sea freezes? (Job 38v28-30)
View all the articles in this series here….
http://bibletruthandprophecy.com/resources/short-articles-archive/creation-not-evolution-articles-by-brother-james-mccann/
or see another proof of creation series here…
http://bibletruthandprophecy.com/resources/short-articles-archive/evidence-of-design-in-the-creation-series/
Visit the dedicated Creation over Evolution area of our site
http://bibletruthandprophecy.com/resources/theistic-evolution-bible-study-resource-area/
Follow us on our dedicated Facebook page
Facebook Page ‘Alethia’ BibleTruthandProphecy
Or our website
BibleTruthandProphecy (Subscribe for updates)
If you would like to subscribe to our YouTube channel, once you have clicked ‘Subscribe’ make sure you click the cog next to the subscribe button and select ‘Send me all notifications for this channel’
Download our ‘Free’ Bible APP – ‘KeyToThe Bible’ for i-phone or Android
Download here…
For more information on the Christadelphians
Read a variety of booklets on-line concerning various key Bible subjects.
Free Bible Booklets